Rearrangement Reactions of Betulin
Rearrangement reactions of betulin have been deeply studied. Researchers have identified, that the most common places to undergo rearrangement reactions are located at the E ring of the betulin scaffold:
- Wagner-Meerwein rearrangement at C(20) position
- Neopentyl rearrangement at C(28) position
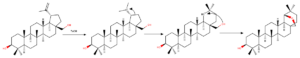
Wagner-Meerwein rearrangement
By treating betulin with strong acid like p-toluenesulfonic acid protonation of C(20)-C(29) double bond takes place. That causes formation of cation at C(20). Further C(21) migration to C(20) causes E ring expansion to form six membered ring. Formed cation in reaction with hydroxyl group at C(28) corresponds to formation of Wagner-Mervein formation product – six membered E ring with 5 membered ether ring – allobetulin – highly active biological compound.
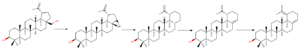
Neopentyl rearrangement
Firstly, to understand rearrangement reactions at C(28) position, lets overlook what is neopentyl rearrangement. Such kind of rearrangement reaction was firstly observed when in hydrolysis reaction of neopenthyliodide (1-bromo-2,2-dimethylpropane) instead of desired product neopenthylalcohol (2,2-dimethylpropan-1-ol) researchers observed rearrangement product – 1,1-dimethylprop-1-ol. That basically should have been nucleophilic substitution, instead rearrangement happened. Explanation of this is that SN2 reaction of such position is highly unfavorable due to steric hindrance. Therefore, reaction has to go with SN1 reaction mechanism, that means, formation of positive cation. As formed cation is primary after rearrangement of methyl group it forms much more stable secondary cation that leads to observed product.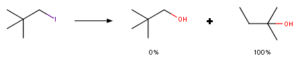
This is the main reason why substitution reactions directly are difficult to archive. Instead aldehyde products can be used to obtain many interesting products overcoming this problem.