Ammonium Glycyrrhizate: Harnessing the Power of Licorice
1. Molecular Identity
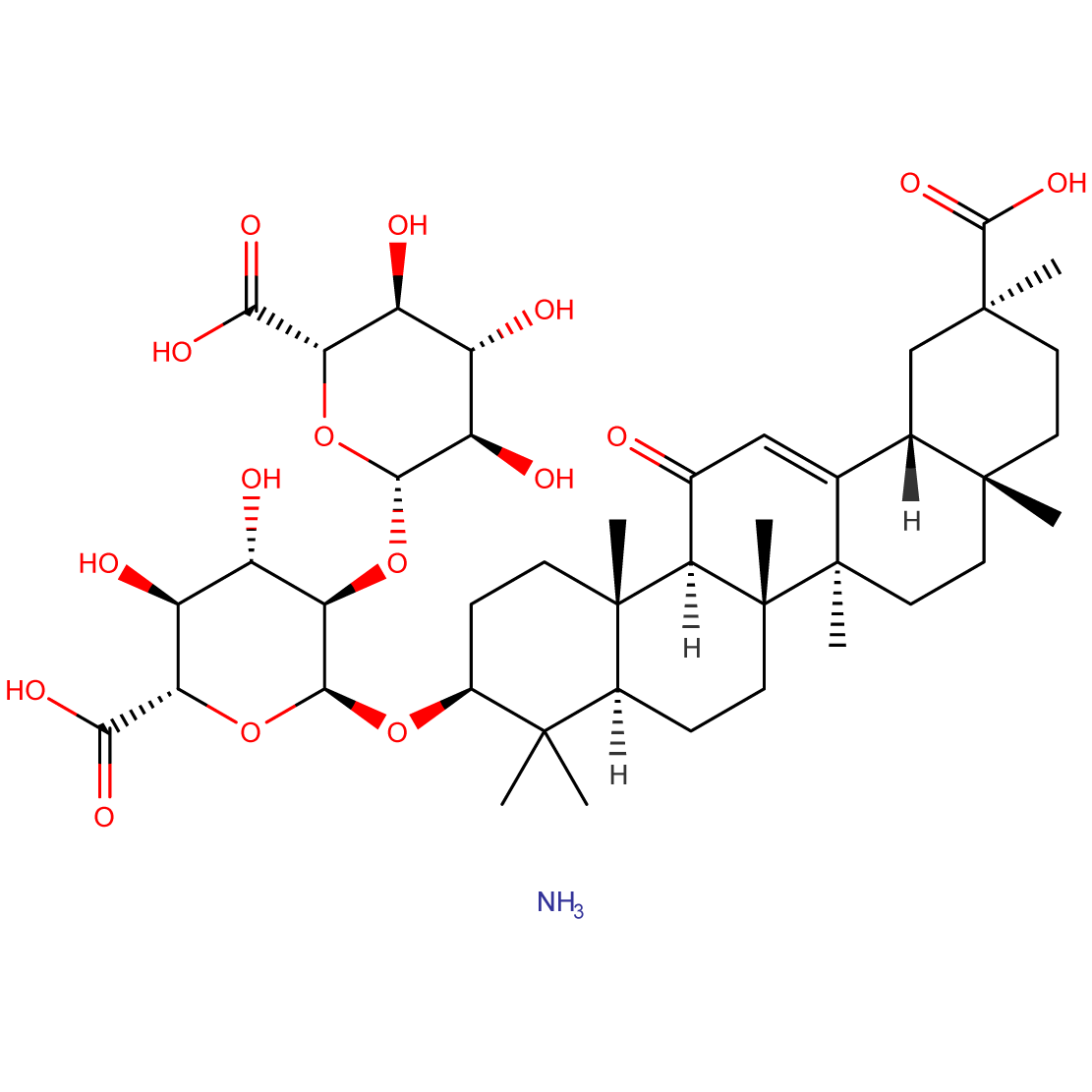
- Chemical Name: Ammonium salt of (3β,20β)-20-carboxy-11-oxo-30-norolean-12-en-3-yl-2-O-β-D-glucopyranuronosyl-α-D-glucopyranosiduronic acid
- CAS Number: 53956-04-0
- Source: Derived from Glycyrrhiza glabra (licorice root)
2. Biochemical Significance
Ammonium Glycyrrhizate is the ammonium salt of glycyrrhizic acid, a major active component of licorice. Its unique molecular structure, combining a triterpenoid backbone with sugar moieties, contributes to its diverse pharmacological activities and widespread applications in pharmaceuticals and food industries.
3. Key Therapeutic Properties
- Anti-inflammatory: Exhibits potent anti-inflammatory effects
- Hepatoprotective: Demonstrates liver-protecting properties
- Antiviral: Shows activity against various viral pathogens
- Sweetening Agent: Provides a natural sweetening effect
4. Potential Applications
- Pharmaceutical research in anti-inflammatory therapies
- Liver disease management studies
- Antiviral drug development
- Natural sweetener in food and beverage industries
5. Current Research Focus
Ongoing studies are investigating Ammonium Glycyrrhizate’s effects on:
- Inflammatory pathways in various disease models
- Liver protection mechanisms against toxic insults
- Viral replication, particularly in hepatitis and influenza
- Taste modification and sugar reduction in food products
6. Formulation Challenges and Innovations
Researchers are actively working on:
- Enhancing bioavailability for systemic applications
- Developing targeted delivery systems for specific organs
- Creating stable formulations for various pharmaceutical and food applications
7. Regulatory Considerations
Ammonium Glycyrrhizate is generally recognized as safe (GRAS) by the FDA for use as a flavoring agent. Its use in specific therapeutic applications would require additional safety and efficacy evaluations to meet regulatory standards.
8. Future Directions
The scientific community anticipates:
- Advanced clinical trials for inflammatory and liver disorders
- Exploration of synergistic effects with other natural compounds
- Development of novel formulations for enhanced efficacy and reduced side effects
9. Collaborative Opportunities
We invite researchers, pharmaceutical companies, and food industry professionals to explore the potential of Ammonium Glycyrrhizate. For inquiries, collaborations, or to discuss how Ammonium Glycyrrhizate can benefit your research or product development, please contact us at sales@nstchemicals.com.
Join us in unlocking the versatile potential of Ammonium Glycyrrhizate – a compound bridging traditional wisdom and modern applications in pharmaceuticals and food science.