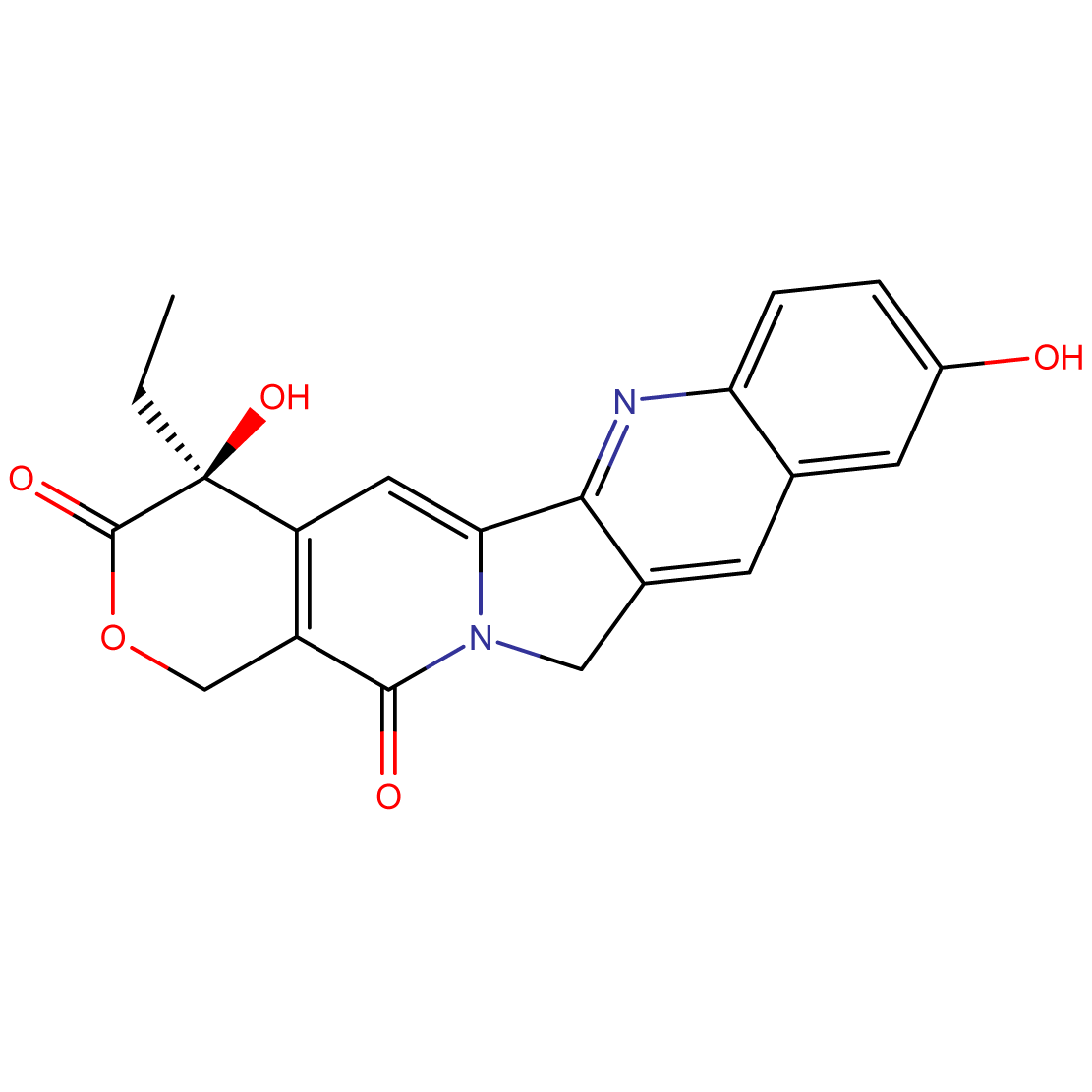
(S)-10-Hydroxycamptothecin: Potent Topoisomerase I Inhibitor for Advanced Cancer Research
1. Molecular Identity
- Chemical Name: (S)-4-ethyl-4-hydroxy-1H-pyrano[3′,4′:6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione
- CAS Number: 19685-09-7
- Source: Semi-synthetic derivative of camptothecin, originally isolated from Camptotheca acuminata
2. Biochemical Significance
(S)-10-Hydroxycamptothecin is a potent topoisomerase I inhibitor with enhanced solubility compared to its parent compound, camptothecin. Its unique structure and mechanism of action make it a compound of significant interest in cancer research and drug development.
3. Key Properties
- Topoisomerase I Inhibition: Potent activity against DNA topoisomerase I
- Anticancer Potential: Exhibits strong cytotoxicity against various cancer cell lines
- Improved Solubility: Enhanced aqueous solubility compared to camptothecin
- S-Configuration: Demonstrates superior biological activity over the R-isomer
4. Potential Research Applications
- Cancer drug development and optimization
- Structure-activity relationship studies of camptothecin analogues
- Investigation of topoisomerase I-mediated DNA damage and repair
- Development of novel drug delivery systems for camptothecin derivatives
5. Current Research Focus
Ongoing studies are investigating (S)-10-Hydroxycamptothecin’s effects on:
- Various cancer types, including drug-resistant tumors
- Combination therapies with other anticancer agents
- Nanoformulations for improved drug delivery
- Mechanisms of resistance to topoisomerase I inhibitors
6. Formulation Challenges and Innovations
Researchers are actively working on:
- Enhancing stability of the active lactone form
- Developing targeted delivery systems for improved efficacy
- Creating prodrug formulations for optimized pharmacokinetics
7. Regulatory Considerations
As a research compound, (S)-10-Hydroxycamptothecin is primarily used in preclinical studies. Its development for therapeutic use would require extensive safety and efficacy evaluations to meet regulatory standards.
8. Future Research Directions
The scientific community anticipates:
- Advanced preclinical and potential clinical trials for specific cancer indications
- Development of novel (S)-10-Hydroxycamptothecin conjugates and analogues
- Exploration of its potential in non-cancer applications, such as antiviral research
9. Collaborative Opportunities
We invite cancer researchers, medicinal chemists, pharmaceutical scientists, and academic institutions to explore the research potential of (S)-10-Hydroxycamptothecin. For inquiries, collaborations, or to discuss how this compound can benefit your research projects, please contact us at sales@nstchemicals.com.
Join us in advancing cancer research with (S)-10-Hydroxycamptothecin – a key player in the development of next-generation topoisomerase I inhibitors.